Iodoform Test for Alcohols
The melting point of PTFE 1 is 600K 327C 620F Low temperature. Uses and environmental effects.

Iodoform Test Description And Mechanism Compounds That Test Positive
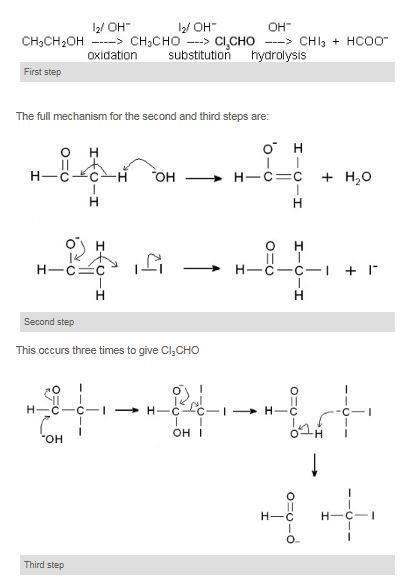
Another useful reaction is used as the iodoform test for methyl ketones which are ketones that have at least one methyl as a functional group.
. Enols are compounds that have a hydroxyl group attached to an unsaturated carbon atom of a double bond. Prepare for BITSAT 2022 with the academic brilliance of ALLEN Leader Online Test Series package which brings to you mock tests for the perfect Abhyas created by our expert faculties. Ratings of chemical behavior listed in this chart apply to a 48 hour exposure period and exposure beyond this timeframe may lead to different impacts.
Alcohols Phenols and Ethers. Aldehydes Ketones and Carboxylic Acids. Di tri tetrachloromethanes iodoform freon and DDT Petroleum.
Alcohols Phenols and Ethers 111 Alcohols. Nylon Chemical Compatibility Chart. JEE Main 2019 Online 10th April Evening Slot.
B From alkyl halide and sodium alkoxide. Chlorhexidine CHX commonly known by the salt forms chlorhexidine gluconate and chlorhexidine digluconate CHG or chlorhexidine acetate is a disinfectant and antiseptic that is used for skin disinfection before surgery and to sterilize surgical instruments. Anhydride of B is u.
Add dilute sodium hydroxide solution drop wise until the brown colour of iodine is. Maintains high strength toughness and self-lubrication as low as 5K -268C -450F and good flexibility at 194K -79C -110F. This test is given by secondary alcohols ketones and acetaldehyde.
It may be used both to disinfect the skin of the patient and the hands of the healthcare providers. Ideal for use with reactive and corrosive chemicals. Physical properties chemical reactions.
Iodoform also known as triiodomethane and. Ii It is because N02 group is electron withdrawing and OCH 3 group is electron releasing. Oxidation of A with KMnO4KOH given acid BC8H6O4.
Or collect it over water and test it with a lighted spill. 103 Uses and environment effects of dichloromethane trichloromethane tetrachloromethane iodoform freons DDT. JEE Mains Chapter wise Practice Questions Last 30 Years with 5000 Questions for online practice.
Cole-Parmer has no knowledge of possible effects beyond this period. CH 3 CH 2 OH and certain secondary alcohols CH 3 CHROH where R is an alkyl or aryl group. Highly resistant to most acids alcohols detergents and solvents.
Chemistry in Everyday Life. Therefore o-nitrophenoxide ion is. Nomenclature methods of preparation physical and chemical properties of primary alcohols only.
Check the chemical compatibility of Nylon with various chemicals solvents alcohols and other productsThere are many forms of Nylon so this is just a broad overview of general compatibility. Add 1ml of 1 iodine solution to it. Enter the email address you signed up with and well email you a reset link.
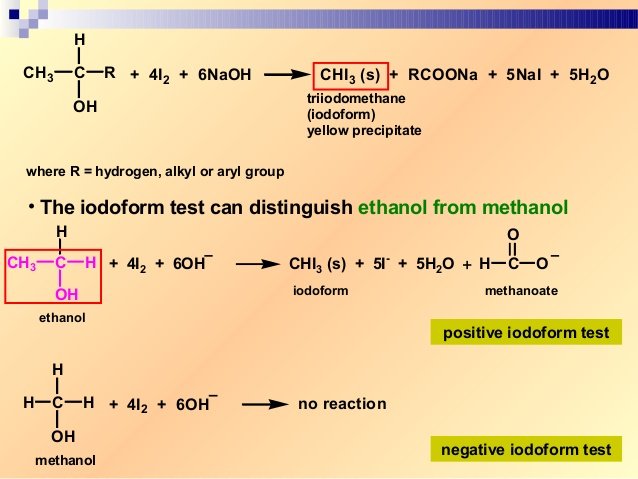
Ethanol when reacted with I2 and NaOH or NaOI gives yellow ppt of iodoform since it has the presence of CH3-CH OH- group. Follow the discussion scenario in sequence for tips to help your students understand a difficult concept in carbon chemistry. The reaction of iodine a base and a methyl ketone gives a yellow precipitate along with an antiseptic smellIt also tests positive for a few specific secondary alcohols that contain at least one methyl group.
Download CBSE Revision Notes for CBSE Class 12 Chemistry Alcohols Phenols and Ethers Alcohols. Students who are preparing for their Class 12 exams must go through NCERT Solutions for Class 12 Chemistry Chapter 10 Haloalkanes and HaloarenesGoing through the solutions provided on this page will. DNPH can also be used to distinguish alcohols and esters from aldehydes and ketones since DNPH does not react with alcohols or esters.
The reaction between zinc powder and. Ratings of chemical behavior listed in this chart apply at a 48-hr exposure period. Haloalkanes And Haloarenes Class 12 NCERT Solutions includes all the important topics with detailed explanation that aims to help students to understand the concepts better.
Take 1ml of given organic compound in a clean dry test tube. Iii Preparation of ethers by acid dehydration of secondary or tertiary alcohols is not a suitable method. The reaction of iodine and base with methyl ketones is so reliable that the iodoform test the appearance of a yellow precipitate.
Iodoform test is used to check the presence of carbonyl compounds with the structure R-CO-CH 3 or alcohols with the structure R-CHOH-CH 3 in a given unknown substance. Before permanent installation test the equipment with the chemicals and under the specific conditions of your application. Here the alkyl halide should be primary and alkoxide should be tertiary.
Ammonia Gas Hot A-Excellent AlcoholsHexyl B-Good Aniline Oil D-Severe Effect AlcoholsIsobutyl A-Excellent Animal Oil NA AlcoholsIsopropyl A-Excellent Argon B-Good AlcoholsMethyl A-Excellent Atomotive Break Fluid A-Excellent AlcoholsOctyl B-Good Automatic Transmission Fluid D-Severe Effect AlcoholsPropyl A-Excellent. Compound AC9H10O shows positive iodoform test. I It is because alcohols can form H-bonds with water molecules whereas hydrocarbons do not.
If your application is more rigorous please request a sample of our EPDM-containing rubber rolls or rubber puzzle tiles in order to test against the chemicals in question. Identification of primary secondary and tertiary alcohols. Cole-Parmer does not warrant neither express nor implied that the.
First the compound is heated with sodium hydroxide solution and iodine. To illustrate the differences in reactivity of isomeric alcohols try using the iodoform demonstration in the video. Alcohols are compounds that have a hydroxyl group left text - OH right attached to a saturated carbon atom.
Nomenclature methods of preparation physical and chemical properties of primary alcohols only identification of primary secondary and tertiary alcohols mechanism of dehydration uses with special reference to methanol and ethanol. We call this lack of reaction a negative result. The information in this chart has been supplied by reputable sources and is to be used ONLY as a guide in selecting equipment for.

A Test To Distinguish Between Ethanol And Methanol Experiment Rsc Education

Iodoform Test Description And Mechanism Compounds That Test Positive

No comments for "Iodoform Test for Alcohols"
Post a Comment